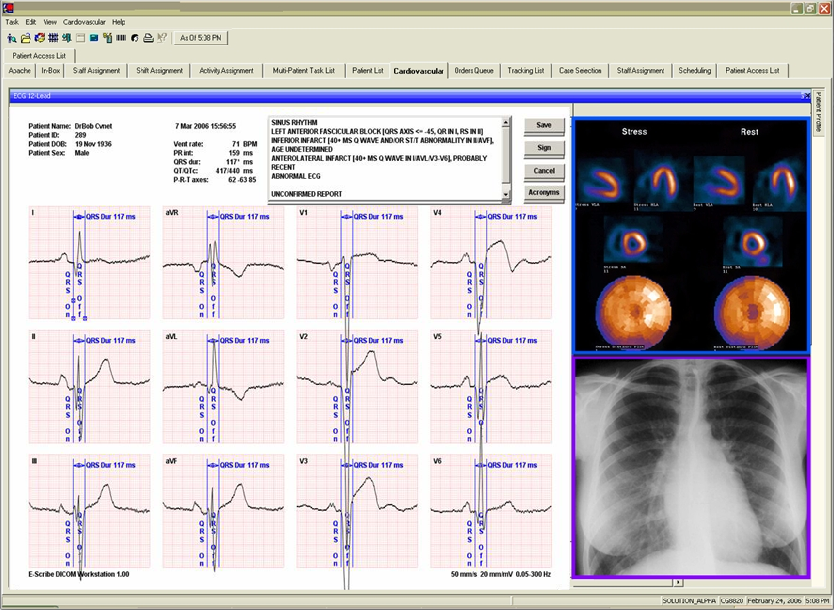
DICOM 12-lead ECG
What do you do when you only have 3% market share? You change the market…
When I joined Mortara Instrument as global product manager over ECG back in 2006, they had approximately 5% of the non-invasive diagnostic ECG market, and perhaps 3% of the non-CRO ECG management systems. At the time, the GE MUSE (Marquette Universal System of Electrocardiography), and the various GE electrocardiograph enjoyed around ~60% market share as the Philips TraceMaster was on the wane in those days. The storage methodology of the day was either XML or Marquette’s proprietary Hilltop protocol.
By storing a 12 lead ECG in DICOM, you could store it in any hospital PACS system that they already had for storing X-Ray, CT, MRI, and other radiology procedures. It really leveled the playing field in the ECG device and ECG storage system marketplace worldwide. Today, manufacturers standardize on DICOM (when they really shouldn’t – see my LinkedIn article on the subject of metadata in DICOM ECG).
That having been said, DICOM 12 Lead ECG won a 2008 Frost and Sullivan Best Practices award for market strategy. I remember at the American College of Cardiology, my old boss at GE ridiculed DICOM 12-lead ECG calling it the “standard of one”. Pretty soon it became the standard of many and put the ability to store non-invasive cardiology data: whether it was a native 12/15 lead ECG, or PDF-encapsulated stress and Holter files. DICOM ECG definitely changed the landscape of non-invasive cardiology storage technology.